Pediatric Pain Measurement
Assessment of Fetal Pain: Evidence from experimental stimulation?
By Nadja Reissland, DPhil, MA, BSc, Cpsychol and Kanwaljeet J.S. Anand, MBBS, DPhil, FAAP, FCCM, FRCPCH
A recent review explored the question of whether analgesia was necessary for fetal procedures and concluded, that “external stimuli can awake a fetus” and therefore “direct fetal analgesia/anaesthesia is mandatory”[1]. These authors also argued that endocrine neuroinhibitors do not keep the fetus in a constant state of sleep as suggested by Mellor, et al. (2005)[2]. Mellor, et. al. posited that endogenous neuroinhibitors, such as adenosine, are present in the placenta and may induce a sleep-like state in the fetus. Immediately after delivery, however, neonates with the same blood levels of neuroinhibitors as those found in the fetus, are often awake and crying when exposed to air. Though the birthing process may cause arousal through widespread neuronal activation[3] it follows that other somatic sensory stimuli (pain) may also cause similar arousal in the fetus. Furthermore, most fetal procedures are performed in utero, without exposure to the birth-related stimulation. The idea that the fetus is kept in a constant sleep state is further contradicted by recent research on fetal reactions to external stimulation. For example, Kiuchi, et al. found that all fetuses in their study reacted to sound but that only those fetuses in states 2F and 3F responded to light[4]. Magnetoencephalographic responses have similarly characterized the fetal responses to sound and light[5-7].
Talking about wakefulness or consciousness is dependent on the definition of these terms. If we agree that the fetus is capable of perceiving and reacting to external stimulation, then this is enough evidence to suggest that the fetus needs anaesthesia, always weighing the risks of prolonged pain/stress against those of analgesia and anaesthesia.
Research on fetal facial movements indicates that fetuses who are neither stimulated nor in pain are capable of coordinating their facial muscles to form a pain expression using facial muscle movements that were associated with pain expression in newborn and premature infants[8]. In this study, six facial movements were identified with combinations of four facial movements expressing painful facies (see Fig 1) as seen in premature infants[9] and relating to the core behavioural cues of infant clinical pain as assessed by the Neonatal Facial Coding System[10].
Further evidence for fetal awareness and fetal reactions to experimental stimulation includes, for example, that fetuses recognize their mothers’ voice and differentiate it from another woman’s voice as expressed in their heart rate changes[11]. More specifically, fetuses react with distinct mouth movements when hearing a particular sound, apparently mirroring the mouth movements necessary to produce the sound silently[12].
Stressed fetuses respond with increases in fetal plasma cortisol concentrations[13] and increased blood flow to the brain after needling of the intrahepatic vein[14]. Maternal stress also had an effect on fetal laterality with decreased right-handed touch evidenced via ultrasound in fetuses of mothers reporting stress[15].
In a review of neurodevelopmental changes observed after painful stimulation in human and non-human fetuses, Lowrey et al. argued that recurring painful stimulation leads to the formation of abnormal synapses resulting in hyperactive responses to pain[16], similar to newborns [17]. Thus, it is important to assess and treat fetal pain. Assessment of fetal pain may include the interpretation of facial expressions[8, 18], body movements[19,20], crying-like activity, as well as the hemodynamic or hormonal changes that occur immediately after tissue injury[21-23].
This brief review points to the fact that human fetuses are at some level capable of pain perception, which varies with fetal gestational age. Given that the research on fetal pain is still in its infancy, we recommend striking a balance between the benefits of pain reduction and survival with unaltered brain development.
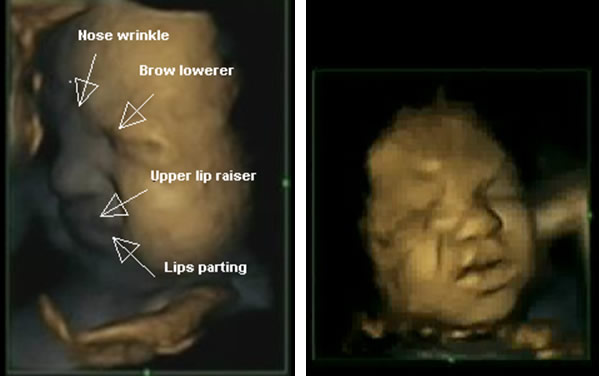
Fig. 1 Fetuses showing ‘pain’ ‘distress’ expressions
References
- Bellieni, C.V., S. Vannuccini, and F. Petraglia, Is fetal analgesia necessary during prenatal surgery? J Matern Fetal Neonatal Med, 2018. 31(9): p. 1241-1245.
- Mellor, D.J., et al., The importance of 'awareness' for understanding fetal pain. Brain Res Brain Res Rev, 2005. 49(3): p. 455-71.
- Tang, L.Q., et al., C-fos gene expression in rat brain around birth: effect of asphyxia and catecholamines. Brain Res, 2000. 852(1): p. 84-91.
- Kiuchi, M., et al., The relationship between the response to external light stimulation and behavioral states in the human fetus: how it differs from vibroacoustic stimulation. Early Hum Dev, 2000. 58(2): p. 153-65.
- Govindan, R.B., et al., An objective assessment of fetal and neonatal auditory evoked responses. Neuroimage, 2008. 43(3): p. 521-7.
- Matuz, T., et al., Habituation of visual evoked responses in neonates and fetuses: a MEG study. Dev Cogn Neurosci, 2012. 2(3): p. 303-16.
- Muenssinger, J., et al., Sensitivity to Auditory Spectral Width in the Fetus and Infant - An fMEG Study. Front Hum Neurosci, 2013. 7: p. 917.
- Reissland, N., B. Francis, and J. Mason, Can healthy fetuses show facial expressions of "pain" or "distress"? PLoS One, 2013. 8(6): p. e65530.
- Anand, K.J.S. and D.B. Carr, The neuroanatomy, neurophysiology, and neurochemistry of pain, stress, and analgesia in newborns and children. Pediatr Clin North Am, 1989. 36(4): p. 795-822.
- DiLorenzo, M.G., et al., Infant Clinical Pain Assessment: Core Behavioural Cues. J Pain, 2018.
- Kisilevsky, B.S., et al., Effects of experience on fetal voice recognition. Psychol Sci, 2003. 14(3): p. 220-4.
- Reissland, N., et al., Do fetuses move their lips to the sound that they hear? An observational feasibility study on auditory stimulation in the womb. Pilot Feasibility Stud, 2016. 2: p. 14.
- Giannakoulopoulos, X., et al., Fetal plasma cortisol and beta-endorphin response to intrauterine needling. Lancet, 1994. 344(8915): p. 77-81.
- Teixeira, J.M., V. Glover, and N.M. Fisk, Acute cerebral redistribution in response to invasive procedures in the human fetus. Am J Obstet Gynecol, 1999. 181(4): p. 1018-25.
- Reissland, N., et al., Laterality of foetal self-touch in relation to maternal stress. Laterality, 2015. 20(1): p. 82-94.
- Lowery, C.L., et al., Neurodevelopmental changes of fetal pain. Semin Perinatol, 2007. 31(5): p. 275-82.
- Gokulu, G., et al., Comparative heel stick study showed that newborn infants who had undergone repeated painful procedures showed increased short-term pain responses. Acta Paediatr, 2016. 105(11): p. e520-e525.
- Reissland, N., What the fetal face can tell us: A discussion of the evidence, implications and potential for further research. Donald School J Ultrasound Obstet Gynecol, 2014. 8(4): p. 336-343.
- Grunau, R.E., et al., Are twitches, startles, and body movements pain indicators in extremely low birth weight infants? Clin J Pain, 2000. 16(1): p. 37-45.
- Holsti, L., et al., Is it painful or not? Discriminant validity of the Behavioral Indicators of Infant Pain (BIIP) scale. Clin J Pain, 2008. 24(1): p. 83-8.
- Bellieni, C.V., Pain assessment in human fetus and infants. AAPS J, 2012. 14(3): p. 456-61.
- Teixeira, J., et al., Fetal haemodynamic stress response to invasive procedures. Lancet, 1996. 347(9001): p. 624.
- Anand, K.J. and M. Maze, Fetuses, fentanyl, and the stress response: signals from the beginnings of pain? Anesthesiology, 2001. 95(4): p. 823-5.


