Efficacy of Intraoperative Dexmedetomidine in Decreasing Morphine Consumption After Scoliosis Surgery in Children
By Silky Wong, MBBS, Thomas Engelhardt, MD, Christian Zaarour, MD, Igor Luginbuehl, MD, Jacqueline Hanley, MN, Cengiz Karsli, MD
Department of Anesthesia, The Hospital for Sick Children, University of Toronto,
Toronto, Ontario, Canada
(Summarized and submitted by Silky Wong, MBBS)
Posterior spinal fusion surgery for idiopathic scoliosis is associated with considerable postoperative pain and opioid side effects. The selective alpha-2 adrenoreceptor agonist dexmedetomidine has been shown to reduce intraoperative remifentanil and propofol requirements. We hypothesized that intraoperative dexmedetomidine decreases postoperative opioid requirements in children.
Following research ethics board approval, the charts of children under 18 years of age who underwent posterior instrumentation for correction of idiopathic scoliosis were reviewed. With the exception of dexmedetomidine (0.5mg/kg/h upon skin incision), all children had a similar anesthetic technique. Full neurophysiologic monitoring was instituted and surgery was performed by the same surgeon in all cases.
Cumulative opioid requirements and pain scores (numerical rating system, 0 to 10) up to 48 hours postoperatively were recorded. All opioids administered were converted to morphine equivalent dosing and compared using the unpaired Student’s t-test. Pain scores were analyzed using two-way repeated measures ANOVA. P<0.05 was considered significant.
Data from 47 (23 group D and 24 group C) children are presented. Mean morphine equivalent consumption on post-operative day (POD) 0, 1 and 2 (up to 48h post-operatively) did not differ between groups.
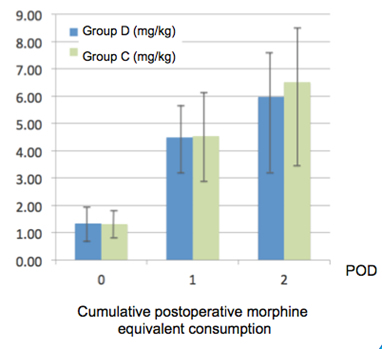
The duration of anesthesia was similar in both groups (445±73.4 min group D and 398±68 min group C). The two groups had similar pain scores throughout the study.
The results of this study suggest that intraoperative dexmedetomidine does not reduce postoperative opioid requirements or pain scores in children undergoing idiopathic scoliosis repair.


